- CDC
- Heart Failure
- Cardiovascular Clinical Consult
- Adult Immunization
- Hepatic Disease
- Rare Disorders
- Pediatric Immunization
- Implementing The Topcon Ocular Telehealth Platform
- Weight Management
- Monkeypox
- Guidelines
- Men's Health
- Psychiatry
- Allergy
- Nutrition
- Women's Health
- Cardiology
- Substance Use
- Pediatrics
- Kidney Disease
- Genetics
- Complimentary & Alternative Medicine
- Dermatology
- Endocrinology
- Oral Medicine
- Otorhinolaryngologic Diseases
- Pain
- Gastrointestinal Disorders
- Geriatrics
- Infection
- Musculoskeletal Disorders
- Obesity
- Rheumatology
- Technology
- Cancer
- Nephrology
- Anemia
- Neurology
- Pulmonology
Ten HIV Clinical Trials That Really Matter
This AIDS specialist offers his rank-order list of the top 10 HIV clinical trials that really made a difference. The trials differ substantially in design, size, and focus, but they all were “game changers.”
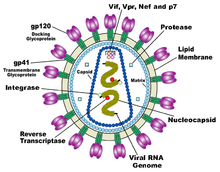
Diagram of HIV virion. (Courtesy, Wikipedia.)
Thirty-three years after the first reports of gay men dying of opportunistic infections appeared in The New England Journal of Medicine, and as we have just participated in the 27th annual World AIDS Day events on December 1, I want to take this opportunity to remember and acknowledge the tens of millions of individuals worldwide who have battled HIV infection along the way.
A generation ago, we knew nothing of the virus that, as of 2012, was estimated to have infected 75 million persons-of whom almost half have died. And as we pause to reflect on the immense personal and economic toll that the virus has had across the globe, it may be worth remembering the progress that has occurred, in large part, because millions of HIV-infected persons cared enough about making a difference that they chose to participate in HIV clinical trials.
I first participated in the care of an HIV-infected person in 1985. I saw my first patient die of HIV-related complications in 1986. It is almost impossible to describe how different the situation was then, compared with now. But in 1986, the year I entered fellowship training in infectious diseases, there were no antiretroviral therapies approved for treating HIV, and infection with HIV was, truly, a “death sentence.” Think Ebola. Fear, desperation, and hopelessness were rampant. That the situation has changed 180 degrees is a result of progress made, in large part, through decades of clinical trials research.
What follows is my rank-order list of the top 10 HIV clinical trials that really made a difference. The trials differ substantially in design, size, and focus, but they all were “game changers.”
Number 10: Efficacy of AZT monotherapy versus placebo for preventing deaths from HIV-related complications.1 This trial led to the FDA approval of AZT in March 1987. AZT monotherapy thus became the first approved therapy for HIV. In the trial, 19 deaths occurred in the placebo arm, compared with 1 in the AZT arm. Five years into the epidemic, we had a glimmer of hope.
Number 9: Concorde.2 In 1993, at the Berlin World Aids Conference, Concorde landed with a resounding thud. The trial concluded that the administration of AZT (versus placebo) to HIV-infected persons who were asymptomatic did not alter progression of HIV disease or survival rates in the medium to long term. I was at that meeting in the summer of 1993. It marked the low point in HIV care and clinical trials research for most of us. Researchers and scientists and, indeed, the entire health care profession, all were criticized for not doing enough, and spending too much time attending off-site “parties” affiliated with international conventions.
Number 8: The safety and efficacy of lamivudine (3TC)/zidovudine combination therapy vs zidovudine monotherapy and lamivudine monotherapy in therapy-naive, HIV-positive patients with CD4 cell counts of 200-500/µL: a randomized, double-blind, multicenter, controlled trial.3 It took us awhile, but in 1995 we published compelling evidence that combination therapy was better at suppressing HIV RNA than was monotherapy. The trial contributing to the data presented in the publication, sponsored by Glaxo Wellcome, was the first large trial to use HIV RNA, instead of CD4+ cell count, as a surrogate for clinical endpoints.
Two other trials in that same era deserve mention: ACTG 1754 and NU-COMBO.5 Both of those trials began earlier than the first one listed, although both were published a year after the first one. I enrolled participants in all 3 trials, but the one I listed as “game changing” is there primarily because it changed the focus of care from immunology-based to virology-based. Subsequent to the publication of the Glaxo-sponsored trial, all of us began thinking about “potency” of antiretrovirals in terms of the sum of the log reduction in HIV RNA that each drug in the “cocktail” provided.
Number 7: Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection.6 This Abbott-sponsored trial, presented in the fall of 2000 in Toronto at an international microbiology meeting, forever changed the way we prescribe HIV protease inhibitors. Prior to that presentation, nelfinavir-a protease inhibitor not combined with ritonavir-was the worldwide market-share leader in sales of antiretrovirals. As a result of this trial, we came to understand that combining a protease inhibitor with small doses of another protease inhibitor (ritonavir, a potent inhibitor of cytochrome P-450) resulted in very high levels of the first protease inhibitor and made it substantially less likely for drug-limiting mutations to be selected.
Number 6: Class-sparing regimens for initial treatment of HIV-1 infection.7 This was ACTG 5142, presented in Toronto at the World AIDS meeting in 2006. The trial randomized participants to therapy with efavirenz, a non-nucleoside reverse transcriptase inhibitor (NNRTI), combined with 2 nucleoside reverse transcriptase inhibtors (NRTIs) versus lopinavir/ritonavir combined with 2 NRTIs versus lopinavir/ritonavir plus efavirenz. The efavirenz arm resulted in the best suppression of HIV RNA, and established the NNRTI class as a very potent (and easy-to-take) option for therapy.
Two other important findings came out of that trial: the combination of the ritonavir-boosted PI with efavirenz resulted in the highest number of side effects, and the ritonavir-boosted PI arm resulted in the best CD4+ cell count increase. I presented another major trial at the same meeting,8 which also showed a higher rate of side effects in the PI plus NNRTI arm. As a result of both trials, guidelines changed to recommend against the use of “triple-class” therapy in previously antiretroviral-naive persons. As for the difference in CD4+ cell counts seen with the different classes, debates about the clinical relevance of this finding (repeated in several subsequent trials) continued for years.
Finally, this fall, my colleagues and I presented data from a meta-analysis of many trials, which showed that there was no difference in HIV RNA suppression, CD4+ cell count increase, or clinical outcome comparing NNRTI-based with PI/r-based regimens.9
Number 5: Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE Study (MTN 003).10 Five thousand non–HIV-infected African women were randomized to 1 of 3 pre-exposure prophylaxis (PrEP) regimens or to placebo. None of the regimens was more effective than placebo at preventing new infections with HIV. Although the participants had excellent self-reported rates of adherence, other more objective measures of adherence found that adherence to PReP was poor. These results, on a large population level, confirm the earlier results of another “failed” PrEP trial (FEM-PrEP),11 and call into question the long-term sustainability of the positive PrEP findings from other trials such as iPrEx.12
Number 4: Prevention of HIV-1 infection with early antiretroviral therapy.13 This is HPTN 052, published in 2011-the trial that randomized 1764 discordant couples worldwide to a strategy of early antiretroviral therapy (ART) for the HIV-infected partner versus delayed ART initiation (waiting until the CD4+ cell count dropped below 250/µL). Of the 28 new infections that were linked to a participating HIV-infected partner, only 1 occurred in the early treatment group. In other words, by suppressing HIV RNA through treating the HIV-infected partners, the HIV-uninfected partners remained substantially protected from acquisition of HIV. The results of this trial have been confirmed subsequently in discordant couples of men who have sex with men (MSM), and form the basis for the current recommendations for “treatment as prevention.”
The basis for these results was found as early as 2000, in a study by Tom Quinn and colleagues,14 which showed that heterosexual transmission of HIV RNA did not occur with HIV RNA levels below 1500 copies/mL.
Number 3: Effect of early versus deferred antiretroviral therapy for HIV on survival.15 This is the large (>17,000 person) NA-ACCORD observational cohort analysis of the effect of treating asymptomatic HIV-infected persons with ART early (CD4+ cell count > 500/µL) versus initiating therapy when the CD4+ cell count was between 350 and 500/µL. The study found that early initiation of ART was associated with improved survival. Published in 2009, it is the only non-randomized (observational) trial that I have included in the top 10 list of the most important HIV clinical trials. This trial resulted in changes in the guidelines, such that initiation of ART is recommended essentially for everyone, regardless of CD4+ cell count.
The NIH is currently funding a large worldwide randomized trial (START) that randomizes asymptomatic HIV-infected persons with CD4+ cell counts >500/µL to immediate initiation of ART versus deferred ART initiation (until the CD4+ cell count drops below 350/µL). Results should be available within another 2 to 3 years.
Number 2: SMART.16 Arguably the most important HIV non-prevention trial ever, this randomized study showed that going off and on ART versus staying on ART resulted in substantially more deaths. Most important-and coming as a complete surprise to almost everyone-a substantial number of the deaths were “non–HIV-related,” such as deaths from cardiac and hepatic causes. In other words, it now is hypothesized that ongoing inflammation from untreated and “clinically asymptomatic” HIV infection results in a state of pro-inflammatory cytokine excess that increases the risk of heart attack and liver and kidney failure. Therapies that reduce inflammation are being investigated by the NIH and other funders; this area of research is one of the “hottest” areas currently in HIV clinical trials.
Number 1: ACTG 076.17 This is the trial that showed that it was possible to substantially reduce mother-to-child transmission of HIV by initiating ART (AZT monotherapy in this trial) in the second or third trimester of pregnancy in HIV-infected women. Several other similar trials, using different antiretrovirals and simpler protocols, confirmed the findings. I can think of no other trial that has had such a profound impact on public health. Published in 1994, the trial changed the “standard of care” for the management of HIV during pregnancy, emphasizing “targeted voluntary screening” for HIV of all pregnant women. As a result, in the United States, we typically have fewer than 50 babies born HIV-infected each year, down from over 1000 in 1992. While not completely understood at the time, the mechanism responsible for this result seems to be ART suppression of HIV RNA to “undetectable levels,” and is another important example of “treatment as prevention.”
So there you have it; my rank-order list of the 10 HIV clinical trials that really mattered. I welcome comments, and would greatly enjoy reading your suggestions for inclusion of other trials not listed in this article.
References:
1. Fischl MA, Richman DD, Grieco MH, et al. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med .1987;317:185-191.
2. Concorde Coordinating Committee. Concorde: MRC/ANRS randomised double-blind controlled trial of immediate and deferred zidovudine in symptom-free HIV infection. Lancet. 1994;343:871-881.
3. Eron J, Benoit S, Jemsek J, MacArthur RD, et al. The safety and efficacy of lamivudine (3TC)/zidovudine combination therapy vs. zidovudine monotherapy and lamivudine monotherapy in therapy-naive, HIV-positive patients with CD4 cell counts of 200-500/mm3: a randomized, double-blind, multicenter, controlled trial. N Engl J Med. 1995;333:1662-1669.
4. Hammer SM, Katzenstein DA, Hughes MD, et al. A trial comparing nucleoside monotherapy with combination therapy in HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. AIDS clinical trials group study 175 study team. N Engl J Med. 1996;335:1081-1090.
5. Saravolatz LD, Winslow DL, Collins G, et al. Zidovudine alone or in combination with didanosine or zalcitabine in HIV-infected patients with the acquired immunodeficiency syndrome or fewer than 200 CD4 cells per cubic millimeter. N Engl J Med. 1996;335:1099-1106.
6. Walmsley S, Bernstein B, King M, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346:2039-2046.
7. Riddler SA, Haubrich R, DiRienzo G, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095-2106.
8. MacArthur RD, Novak RM, Peng G, et al. A comparison of three highly active antiretroviral treatment strategies consisting of non-nucleoside reverse transcriptase inhibitors, protease inhibitors, or both in the presence of nucleoside reverse transcriptase inhibitors as initial therapy (CPCRA 058 FIRST Study): a long-term randomised trial. Lancet. 2006;368:2125-2135.
9. Borges AH, Lundh A, Tendal B, et al. Systematic review and meta-analysis of randomized controlled trials (RCTs) comparing initial non-nucleoside reverse-transcriptase inhibitor (NNRTI)- versus ritonavir boosted protease inhibitor (PI/r)-based anti-retroviral therapy (ART). IDWeek 2014. October 8 – 12, 2014. Philadelphia. Presentation number 536.
10. Marrazzo J, Ramjee G, Nair G, et al. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE Study (MTN 003). 20th Conference on Retroviruses and Opportunistic Infections. March 3 – 6, 2013. Atlanta. Abstract 26LB.
11. Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411-422.
12. Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
13. Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493-505.
14. Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921-929.
15. Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815-1826.
16. The Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283-2296.
17. Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med. 1994;331:1173-1180.
Common Side Effects of Antiretroviral Therapy in HIV Infection
February 7th 2013What are some of the more common side effects of antiretroviral therapy, and what can the primary care physician do to help manage these effects? In this podcast, infectious disease expert Rodger MacArthur, MD, offers insights and points readers to updated comprehensive guidelines.
