- CDC
- Heart Failure
- Cardiovascular Clinical Consult
- Adult Immunization
- Hepatic Disease
- Rare Disorders
- Pediatric Immunization
- Implementing The Topcon Ocular Telehealth Platform
- Weight Management
- Monkeypox
- Guidelines
- Men's Health
- Psychiatry
- Allergy
- Nutrition
- Women's Health
- Cardiology
- Substance Use
- Pediatrics
- Kidney Disease
- Genetics
- Complimentary & Alternative Medicine
- Dermatology
- Endocrinology
- Oral Medicine
- Otorhinolaryngologic Diseases
- Pain
- Gastrointestinal Disorders
- Geriatrics
- Infection
- Musculoskeletal Disorders
- Obesity
- Rheumatology
- Technology
- Cancer
- Nephrology
- Anemia
- Neurology
- Pulmonology
HIV-Associated Nemaline Rod Myopathy: Role of Intravenous Immunoglobulin Therapy in Two Persons With HIV/AIDS
Nemaline rod myopathy is a rare human neuromuscular disorder defined by the presence of rod-shaped structures infiltrating muscle fibers.
Nemaline rod myopathy is a rare human neuromuscular disorder defined by the presence of rod-shaped structures infiltrating muscle fibers. Also known as rod body disease, it was identified in 1958 by Dr Douglas Reye.1 In 1963, 2 separate groups published the initial reports of this condition.2,3 It was only after Reye’s original description of rodlike fragments in the myofibrils of a 3-year-old boy that this condition was considered congenital. The mechanism of disease involves certain genetic alterations that trigger the formation and deposit of proteinaceous material in skeletal muscle. This muscular rod infiltration may result in weakness, a known characteristic of nemaline rod myopathy.
There are also several cases in the literature of acquired illnesses resulting in nemaline rod myopathy, and there appears to be a strong association with HIV infection.4,5 In the presence of HIV, the virus itself may trigger reactions that cause de novo genetic mutations resulting in nemaline formation.6-11 Other conditions associated with the secondary formation of nemaline bodies include monoclonal gammopathy, polymyositis, hypothyroidism, muscular dystrophy, and quadriplegic myopathy.12-14 Nemaline rods have also been observed in the muscle of patients receiving hemodialysis and in patients who received radiation therapy or were exposed to β-adrenergic agents (Table).15-18
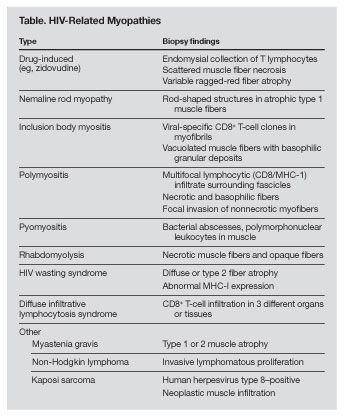
Nemaline rod myopathy has an estimated incidence of 2 per 100,000 live births in its congenital form, although it has been reported to be higher in some populations. In the Ashkenazi Jewish community, a genetically homogenous subgroup, there is a reported 1:108 carrier frequency.17,19,20 There are no statistics describing the incidence or prevalence of nemaline rod myopathy in HIV-infected persons. There is no proven cure for nemaline rod myopathy; therapeutic approaches rely heavily on anecdotal reports. We report here 2 cases of nemaline rod myopathy in HIV-infected persons who were successfully treated with intravenous immunoglobulin (IVIG) therapy.
CASE SUMMARIES
Case 1
A 46-year-old white, homosexual man presented with an acute retroviral syndrome, and a diagnosis of HIV infection was made. His HIV RNA level was 209,396 copies/mL and his CD4+ cell count was 350/µL. The patient initially presented with acute HIV myopathy with profound proximal muscle weakness on physical examination. The patient required assistance to stand up, could not raise his arms, and had difficulty in holding his head up; however, he had good strength in his hands and forearms. The patient reported no family history of musculoskeletal diseases. His creatine kinase level was mildly elevated at 373 U/L (normal, 55 to 170). His lactate dehydrogenase level was 667 U/L (normal, 98 to 192), and the aldolase level was normal.
The patient was treated with prednisone and 2 courses of monthly IVIG, which yielded an excellent response with recovery of muscle strength and range of motion to the patient’s baseline, and he was able to resume his 3 weekly weight-lifting sessions. NNRTI-based antiretroviral therapy with efavirenz plus zidovudine and lamivudine was started. At 8 weeks of treatment, the patient’s HIV RNA level was undetectable (less than 50 copies/mL) and his CD4+ cell count had risen above 500/µL.
As of August 2004, the patient had been asymptomatic for 4 years, and he discontinued his antiretroviral therapy. Six months later, he noticed proximal muscle weakness. His HIV RNA level had risen to 179,110 copies/mL; his CD4+ cell count was 518/µL; and his previous antiretroviral regimen was re-initiated. Serum immunoelectrophoresis detected small k chains, while aldolase and creatine kinase levels remained within normal limits. Despite the patient’s good virological response to antiretroviral therapy, he experienced progressive muscle weakness. An electromyogram showed evidence of fibrillation. An MRI scan of the left shoulder revealed inflammation in the muscle. An MRI-guided muscle biopsy showed nemaline rods, many atrophic and nonatrophic fibers, and ragged red myofibrils (Figures 1, 2, and 3).
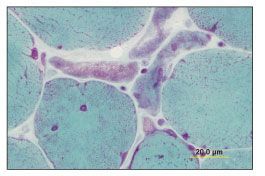
Figure 1.Muscle fibers vary from 6 to 90 µm in diameter. Fibers smaller than 25 µm occur singly or in groups, with up to 4 fibers in some groups (Gomori trichrome stain, original magnification ×152). (Photograph courtesy of Dr Andrew Engel, Mayo Clinic, Rochester, Minn.)
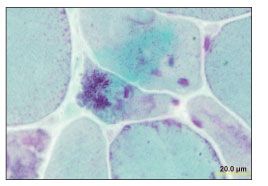
Figure 2.Atrophic fibers filled with nemaline rods, and other nonatrophic fibers containing small vacuolated collections (Gomori trichrome stain, original magnification ×152). (Photograph courtesy of Dr Andrew Engel, Mayo Clinic, Rochester, Minn.)
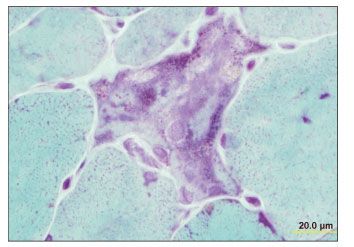
Figure 3.Focal basophilia, vesicular nuclei, small vacuoles, and nemaline rods (Gomori trichrome stain, original magnification ×152). (Photograph courtesy of Dr Andrew Engel, Mayo Clinic, Rochester, Minn.)
After several weeks of corticosteroid therapy (prednisone 40 mg/d), the patient continued to report muscle weakness, with decreased muscle tone in his arms and bilateral pectoralis muscle atrophy. Monthly IVIG infusions were started at a dose of 1 g/kg. In 3 months, his overall clinical condition markedly improved, and his muscle strength had improved dramatically and no deficits were apparent. At this point, his HIV RNA level was again undetectable and his CD4+ cell count was 710/µL. Corticosteroid therapy was tapered off. As of April 2007, the patient remained asymptomatic.
Case 2
A 49-year-old white, homosexual man presented with bilateral shoulder pain, weakness, and decreased range of motion. He tested negative for HIV infection, and a 6-week course of physical therapy was prescribed. During the first month of therapy, the patient noticed difficulty in ambulation secondary to bilateral hip discomfort and decreased thigh strength. This progressed to an inability to stand up or climb stairs.
One year after onset of symptoms, he tested positive for HIV; his initial HIV RNA level was 1144 copies/mL and CD4+ cell count was 600/µL. An electromyogram showed evidence of a moderately severe generalized myopathy, with scattered fibrillation potentials and small polyphasic units. These findings were highly suggestive of myonecrosis, vacuolization, or splitting. A deltoid muscle biopsy specimen revealed nemaline rods and type 1 atrophic fibers with foci of inflammation and regeneration. There was substantial variation in the myofibers’ size and shape, with many displaying increased internal nuclei. No ragged red fibers were noted.
A diagnosis of late-onset nemaline rod myopathy was made. Treatment was started with prednisone (50 mg/d) and IVIG infusions (2 g/kg/d) for 5 days. Antiretroviral therapy was initiated with efavirenz plus tenofovir and emtricitabine. His initial IVIG treatment was followed by monthly infusions at the 2 g/kg dose. In 3 months, his symptoms markedly improved, with muscle strength returning to baseline, and he was able to return to work. His HIV RNA level was undetectable (less than 50 copies/mL). He continued to receive monthly IVIG therapy for 7 months. Corticosteroid therapy was tapered off. He was asymptomatic up to July 2004 when he was lost to follow-up.
DISCUSSION
Nemaline rod myopathy is a rare condition. Its inheritance pattern is autosomal, most frequently recessive.21,22 Isolated cases without family history usually have de novo dominant mutations.23-26 The hereditary form is associated with multiple mutations affecting the nebulin, α-actinin, α-tropomyosin, β-tropomyosin, and troponin T1 genes.22-25 These mutations result in the formation of nemaline bodies, protein-ic material derived from thin filaments and the Z disk of sarcomeres.16 The nemaline bodies can be located inside the nucleus or in the sarcolemma and appear red on optical microscopy using the Gomori technique.27 Hallmark findings on electron microscopy include sarcomeric disruption and glycogen accumulation.28 There is selective type 1 muscle fiber atrophy and alterations in oxidative reactions.29,30 In its congenital form, inflammatory changes are either absent or very mild. Acquired cases of nemaline rod myopathy with inflammatory infiltrates have been described.5,15,31
Nemaline rod myopathy is classified based on its clinical presentation in early (congenital) or late (acquired) onset.13,21,32 The early-onset form is subclassified into severe, intermediate, and typical neonatal syndromes.25 Severe forms present with marked hypotonia and respiratory insufficiency; most fetuses die in utero or within days after delivery. Characteristics of the intermediate form include failure to breathe independently, sit, or ambulate. Ultimately, contractures develop and affected children are wheelchair-bound by age 11.21-25 Common features of the typical form are marked muscular weakness, feeding impairment, and cardiac affectation.17,27
A fourth variant (childhood or juvenile onset) has progressive muscular degeneration, rendering those affected wheelchair-bound by the age of 40.21 The late-onset variant affects adults after the age of 40 with a slow progression of symptoms. Typical symptoms include proximal limb weakness, dropped head, cramping, and muscle pain. Additional findings are areflexia, lax ligaments, and skeletal deformities.4,13 Ventilatory insufficiency secondary to rod infiltration of the respiratory muscles can be fatal.4,25
The exact pathophysiology of nemaline rod myopathy is unknown. An HIV-mediated genome disturbance has been suggested.32 It is likely that HIV infection triggers an immunological response for a de novo mutation.9 Likewise, it is also possible that these alterations represent an epiphenomenon in relation to the HIV infection itself. There is a distinct muscle biopsy pattern in HIV-infected persons with nemaline rod myopathy, consisting of smaller and more punctuated nemaline structures with different degrees of myofibril affectation, from minimal involvement to overt degeneration.32 Severely affected fibers are small and occasionally vacuolated. There is no association between the degree of muscular rod infiltration and the severity of the disease.33,34 Our patients’ myopathic findings correlate with the patterns reported above.
In nemaline rod myopathy, creatine kinase levels can be normal or slightly elevated.4,17 Additional diagnostic methods, such as MRI and electromyography, have shown benefit in diagnosing muscle diseases.17,35-40 In the congenital variant, MRI can be used to localize and characterize the distribution of the involved muscle.36-38 Similarly, this imaging technique can be used to identify affected muscle for biopsy, because these areas may have a patchy distribution.39,40 It may be important to perform MRI-guided biopsy to avoid misdiagnosis or false-negative results. Electromyographic studies usually show a myopathic fibrillation pattern.4,7 Pulmonary function tests and polysomnography may reveal the extent of respiratory compromise.25
Important differences exist between nemaline rod myopathy and zidovudine-induced myopathy, in which the serum creatine kinase level may be elevated up to 10 times with a normal electromyographic pattern.4,7 With zidovudine-induced myopathy, symptoms subside after discontinuation of the medication. The patient in the first case described here had a mildly elevated creatine kinase level with a myopathic electromyographic pattern. His muscle weakness developed 6 months after zidovudine was discontinued, which made the diagnosis of drug-induced myopathy unlikely.
There is no available effective treatment for nemaline rod myopathy. Corticosteroids and other immunosuppressive agents have been used with modest success, pointing toward a possible dysimmune state in nemaline rod myopathy.4-6,41 Plasmapheresis has been reported to be beneficial in cases of nemaline rod myopathy associated with monoclonal gammopathy, indicative of autoimmunity.4,13,18,41 Previously, IVIG therapy had not been used to treat nemaline rod myopathy in HIV-infected persons.
Based on the suspected immunopathic nature of acquired nemaline rod myopathy, administration of IVIG posed a theoretical therapeutic benefit for our patients.4,6,13,42 Successful immunomodulation has been described in patients with hypogammaglobulinemia and as adjuvant therapy to prevent infections in persons with AIDS.8 In our experience, the clinical response to IVIG therapy supports the notion that secondary nemaline rod myopathy may have immunopathic and inflammatory components.43,44 Both our patients had pathological features atypical for the genetic forms of nemaline rod myopathy, which are also known to be poorly responsive to treatment. We hypothesize that IVIG may successfully alter the natural course of HIV-related nemaline rod myopathy, although it is likely that the antiretroviral treatment plays an important role as well. The impact of highly active antiretroviral therapy on this condition has not been assessed because most reported cases in the literature were published before the HAART era.9,45 Future research is required to better understand the underlying immune phenomena of this disease and to assess the role of immunoglobulins as a therapeutic alternative.
Acknowledgment: We would like to thank Dr Andrew Engel from the Mayo Clinic for the processing of the muscle biopsy pictures.
No potential conflict of interest relevant to this article was reported by the authors.
Editorial Comment: HIV-Associated Adult-Onset Nemaline Myopathy
Myopathies are occasionally the only manifestation of HIV infection, in the form of polymyositis (myalgia, muscle weakness, and increased serum levels of muscle enzymes) or muscle fatigue. Muscular complications of HIV infection include polymyositis, inclusion body myositis, nemaline myopathy, diffuse infiltrative lymphocytosis syndrome (DILS), HIV wasting syndrome, vasculitis, myasthenic syndrome and chronic fatigue, toxic drug effects (eg, zidovudine-related mitochondrial myopathy), HIV-associated lipodystrophy and immune restoration syndromes, opportunistic infections and tumor infiltrations of skeletal muscle, and rhabdomyolysis.1,2 de Sanctis and colleagues3 present 2 interesting cases of patients with HIV-associated adult-onset nemaline rod myopathy. The clinical conditions of these 2 patients improved following intravenous immunoglobulin (IVIG) therapy.
Nemaline myopathy is an uncommon disorder characterized by the presence of rodlike inclusions called nemaline bodies (in Greek nema = thread) in myofibers. These nemaline bodies are composed of actin and α-actinin.4 The presence of nemaline rods alone in an adult is insufficient for a diagnosis of adult-onset nemaline myopathy (AONM), because these structures may be present in a number of other neuromuscular disorders, including polymyositis, mitochondrial myopathies, acute alcoholic myopathy, mixed myopathies, and progressive spinal muscular atrophy.5 Nemaline rods have also been identified in patients with no skeletal muscle symptoms. Therefore, a diagnosis of AONM can only be made in a symptomatic patient if large numbers of nemaline rods are evident in the absence of findings typically seen in other myopathies.
HIV-associated AONM was first reported in 1987 in a 26-year-old man who presented with proximal muscle weakness and severe muscle wasting as the initial manifestation of his underlying HIV infection.6 Since then, at least 10 additional cases of HIV-associated AONM have been described in the literature (Table).6-12 Similar to the 2 patients described by de Sanctis and colleagues, there appears to be a male preponderance, and with near uniformity HIV-associated AONM patients have sought medical attention for evaluation of striking proximal muscle weakness and atrophy. The factors that contribute to HIV-associated AONM are uncertain. Patients may possess well-preserved CD4+ cell counts, and in our own experience, patients’ HIV RNA levels may be less than 75 copies/mL.
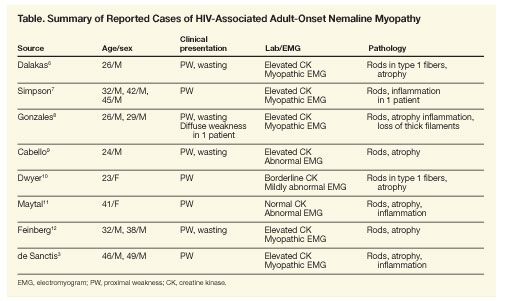
The clinical picture of subacutely evolving muscle weakness and characteristic muscle histology and no other reasons for myopathy point to a diagnosis of AONM. Results of laboratory studies may show normal or elevated creatine kinase levels. Electromyographic (EMG) findings suggest a myopathic process with brief, low-amplitude, polyphasic motor unit potentials and fibrillation potentials. Under light microscopy, the frequency of rod-bearing fibers varies between patients and between different muscles of the same patient. The rods are best observed in trichrome sections or after immunostaining with α-actinin or myotilin. Larger abnormal fibers contain small clusters of rods, whereas in atrophic fibers, rods clearly predominate. Type 1–fiber (slow-twitch oxidative fiber) preponderance is observed in some cases. Minor inflammatory changes may also be present.13 Electron microscopy shows the rods originating from the Z disks, and they may have a characteristic periodicity. In persons with congenital nemaline myopathy, an MRI scan shows selective involvement of the muscles with patchy, fatty degeneration.14 MRI findings in AONM have not been well described.
The association of AONM with monoclonal gammopathy, viral infection (most notably HIV and human T-cell lymphotropic virus type 2 infections), and primary hypothyroidism coupled with the presence of small inflammatory infiltrates on muscle biopsy specimens suggest a role for paraproteins, immunodeficiency, or autoimmunity in the pathogenesis of this condition.5 HIV-1 infection may alter the host genome or posttranslational events in the myocytes and increase the production of a Z band–like material.7
Despite the occasional reports of patient improvement or stabilization following treatment with immunosuppressive regimens, the data remain sparse and the precise details regarding diagnosis and treatment have not been well documented. In a case series published by Chahin and colleagues,13 4 of 8 patients with AONM who received prednisone alone or in combination with other immunosuppressants (methotrexate, cyclophosphamide, or IVIG) improved or stabilized; 3 of the nonresponders died within 1 year. Patients with HIV-associated AONM have also improved following treatment with corticosteroids and plasmapheresis.7,8,10 Unfortunately, good clinical results are not the norm. A recent report describes a patient with AONM and monoclonal gammopathy who was treated with prednisone, IVIG, and rituximab-all interventions without benefit.15
Assessment of the HIV-infected patient who complains of proximal muscle weakness and who has muscle atrophy should include laboratory studies consisting of the following: erythrocyte sedimentation rate; creatine kinase, aldolase, thyroid hormone, and serum lactate levels; antinuclear antibody and rheumatoid factor; and serum protein electrophoresis. EMG and nerve conduction velocity studies may also be useful in serving to characterize the myopathy. A muscle biopsy is an essential component if the cause of myopathy remains uncertain. Given the rarity of HIV-associated AONM, referral to a neurologist or rheumatologist with expertise in HIV-related musculoskeletal disorders is important, as is an outside review of the muscle biopsy by a pathologist who has knowledge of this uncommon condition. Nutritional services, physical and occupational therapy, and complementary approaches are also important components to the comprehensive care of patients with HIV-associated AONM.
The dearth of therapeutic modalities of proven benefit for AONM makes it difficult for health care providers to offer definitive recommendations to affected patients. In this context, the response to IVIG reported by de Sanctis and colleagues opens another avenue of opportunity for treating this very rare, but disabling and potentially fatal, disorder.
Deepthi Mani, MD
Visiting Physician
Division of Hematology and Oncology, Virginia Mason Clinic
Seattle
David M. Aboulafia, MD
Attending Hematologist and Oncologist
Division of Hematology and Oncology, Virginia Mason Clinic
Medical Codirector
Bailey Bouchay House
Clinical Professor of Medicine
Division of Hematology, University of Washington
Seattle
References1. Authier FJ, Chariot P, Gherardi RK. Skeletal muscle involvement in human immunodeficiency virus (HIV)-infected patients in the era of highly active anti-retroviral therapy (HAART). Muscle Nerve. 2005;32:247-260.
2. Sheikh RA, Yasmeen S, Munn R, et al. AIDS-related myopathy. Med Electron Microsc. 1999;32:79-86.
3. de Sanctis JT, Cumbo-Nacheli G, Dobbie D, Baumgartner D. HIV-associated nemaline rod myopathy: role of intravenous immunoglobulin therapy in two persons with HIV/AIDS. AIDS Reader. 2008;18:92-96, 102-103.
4. Yamaguchi M, Robson RM, Stromer MH, et al. Nemaline myopathy rod bodies. Structure and composition. J Neurol Sci. 1982;56:35-56.
5. Gyure KA, Prayson RA, Estes ML. Adult-onset nemaline myopathy: a case report and review of the literature. Arch Pathol Lab Med. 1997;121:1210-1213.
6. Dalakas MC, Pezeshkpour GH, Flaherty M. Progressive nemaline (rod) myopathy associated with HIV infection. N Engl J Med. 1987;317:1602-1603.
7. Simpson DM, Bender AN. Human immunodeficiency virus-associated myopathy: analysis of 11 patients. Ann Neurol. 1988;24:79-84.
8. Gonzales MF, Olney RK, So YT, et al. Subacute structural myopathy associated with human immunodeficiency virus infection. Arch Neurol. 1988;45:585-587.
9. Cabello A, Martnez-Martn P, Gutirrez-Rivas E, Madero S. Myopathy with nemaline structures associated with HIV infection. J Neurol. 1990;237:64-65.
10. Dwyer BA, Mayer RF, Lee SC. Progressive nemaline (rod) myopathy as a presentation of human immunodeficiency virus infection. Arch Neurol. 1992;49:440.
11. Maytal J, Horowitz S, Lipper S, et al. Progressive nemaline rod myopathy in a woman coinfected with HIV-1 and HTLV-2. Mt Sinai J Med. 1993;60:242-246.
12. Feinberg DM, Spiro AJ, Weidenheim KM. Distinct light microscopic changes in human immunodeficiency virus-associated nemaline myopathy. Neurology. 1998;50:529-531.
13. Chahin N, Selcen D, Engel AG. Sporadic late onset nemaline myopathy. Neurology. 2005;65:1158-1164.
14. Wallgren-Pettersson C, Kivisaari L, Jskelinen J, et al. Ultrasonography, CT, and MRI of muscles in congenital nemaline myopathy. Pediatr Neurol. 1990;6:20-28.
15. Keller CE, Hays AP, Rowland LP, et al. Adult-onset nemaline myopathy and monoclonal gammopathy. Arch Neurol. 2006;63:132-134.
No potential conflict of interest relevant to this commentary was reported by the authors.
References:
References1. Schnell C, Kan A, North KN. “An artefact gone awry”: identification of the first case of nemaline myopathy by Dr R.D.K. Reye. Neuromuscul Disord. 2000;10:307-312.
2. Shy GM, Engel WK, Somers JE, Wanko T. Nemaline myopathy. A new congenital myopathy. Brain. 1963;86:793-810.
3. Conen PE, Murphy EG, Donohue WL. Light and electron microscopic studies of “myogranules” in a child with hypotonia and muscle weakness. Can Med Assoc J. 1963;89:983-986.
4. Chahin N, Selcen D, Engel AG. Sporadic late onset nemaline myopathy. Neurology. 2005;65:1158-1164.
5. Miro O, Grau JM, Pedrol E. Distinct light microscopic changes in HIV-associated nemaline myopathy. Neurology. 1999;53:239.
6. Dalakas MC, Pezeshkpour GH, Flaherty M. Progressive nemaline (rod) myopathy associated with HIV infection. N Engl J Med. 1987;317:1602-1603.
7. Maytal J, Horowitz S, Lipper S, et al. Progressive nemaline rod myopathy in a woman coinfected with HIV-1 and HTLV-2. Mt Sinai J Med. 1993;60:242-246.
8. Fontana L, Castro C, Mullen M. Intravenous gammaglobulin in the prevention of infections in severely immunocompromised AIDS patients. 1997 International Scientific Assembly of the American College of Chest Physicians; 1997; New Orleans. Abstract 14S.
9. Colmegna I, Koehler JW, Garry RF, Espinoza LR. Musculoskeletal and autoimmune manifestations of HIV, syphilis and tuberculosis. Curr Opin Rheumatol. 2006;18:88-95.
10. Sheikh RA, Yasmeen S, Munn R, et al. AIDS-related myopathy. Med Electron Microsc. 1999;32:79-86.
11. Cabello A, Martinez-Martin P, Gutierrez-Rivas E, Madero S. Myopathy with nemaline structures associated with HIV infection. J Neurol. 1990;237:64-65.
12. Ginanneschi F, Mondelli M, Malandrini A, et al. Nemaline myopathy: description of an adult onset case. J Submicrosc Cytol Pathol. 2002;34:105-108.
13. Keller CE, Hays AP, Rowland LP, et al. Adult-onset nemaline myopathy and monoclonal gammopathy. Arch Neurol. 2006;63:132-134.
14. Pavlu J, Carey MP, Winer JB. Hypothyroidism and nemaline myopathy in an adult. J Neurol Neurosurg Psychiatry. 2006;77:708-709.
15. Kiernan MC, Bullpitt P, Chan JH. Mitochondrial dysfunction and rod-like lesions associated with administration of beta-2 adrenoceptor agonist formoterol. Neuromuscul Disord. 2004;14:375-377.
16. Portlock CS, Boland P, Hays AP, et al. Nemaline myopathy: a possible late complication of Hodgkin’s disease therapy. Hum Pathol. 2003;34:816-818.
17. North KN, Laing NG, Wallgren-Pettersson C. Nemaline myopathy: current concepts. The ENMC International Consortium and Nemaline Myopathy. J Med Genet. 1997;34:705-713.
18. Eymard B, Brouet JC, Collin H, et al. Late-onset rod myopathy associated with monoclonal gammopathy. Neuromuscul Disord. 1993;3:557-560.
19. Anderson SL, Ekstein J, Donnelly MC, et al. Nemaline myopathy in the Ashkenazi Jewish population is caused by a deletion in the nebulin gene. Hum Genet. 2004;115:185-190.
20. Bruno C, Minetti C. Congenital myopathies. Current Neurol Neurosci Rep. 2004,4:68-73.
21. Sharma MC, Gulati S, Atri S, et al. Nemaline rod myopathy: a rare form of myopathy. Neurol India. 2007;55:70-74.
22. Pelin K, Hilpela P, Donner K, et al. Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc Natl Acad Sci U S A. 1999;96:2305-2310.
23. Schroder JM, Durling H, Laing N. Actin myopathy with nemaline bodies, intranuclear rods, and a heterozygous mutation in ACTA1 (Asp154Asn). Acta Neuropathol (Berl). 2004;108:250-256.
24. Corbett MA, Akkari PA, Domazetovska A, et al. An alpha-tropomyosin mutation alters dimer preference in nemaline myopathy. Ann Neurol. 2005;57:42-49.
25. Ryan MM, Schnell C, Strickland CD, et al. Nemaline myopathy: a clinical study of 143 cases. Ann Neurol. 2001;50:312-320.
26. Ilkovski B, Cooper ST, Nowak K, et al. Nemaline myopathy caused by mutations in the muscle alpha-skeletal-actin gene. Am J Hum Genet. 2001;68:1333-1343.
27. Botelho CH, Carod-Artal FJ, Kalil RK. Nemaline congenital myopathy: clinical features and histopathological findings in nine patients. Rev Neurol. 2001;32:309-314.
28. Nonaka I, Ishiura S, Arahata K, et al. Progression in nemaline myopathy. Acta Neuropathol (Berl). 1989;78:484-491.
29. Miro O, Masanes F, Pedrol E, et al. A comparative study of the clinical and histological characteristics between classic nemaline myopathy and that associated with the human immunodeficiency virus. Med Clin (Barc). 1995;105:500-503.
30. Huang CC, Lee CC, Chen SS. Fiber type disproportion in nemaline myopathy. Zhonghua Yi Xue Za Zhi. 1989;43:229-232.
31. Gyure KA, Prayson RA, Estes ML. Adult-onset nemaline myopathy: a case report and review of the literature. Arch Pathol Lab Med. 1997;121:1210-1213.
32. Feinberg DM, Spiro AJ, Weidenheim KM. Distinct light microscopic changes in human immunodeficiency virus-associated nemaline myopathy. Neurology. 1998;50:529-531.
33. Shimomura C, Nonaka I. Nemaline myopathy: comparative muscle histochemistry in the severe neonatal, moderate congenital, and adult-onset forms. Pediatr Neurol. 1990;6:66.
34. Ryan MM, Ilkovski B, Strickland CD, et al. Clinical course correlates poorly with muscle pathology in nemaline myopathy. Neurology. 2003;60:665-673.
35. Faingold R, Oudjhane K, Armstrong DC, Albuquerque PA. Magnetic resonance imaging of congenital, inflammatory, and infectious soft-tissue lesions in children. Top Magn Reson Imaging. 2002;13:241-261.
36. Scott DL, Kingsley GH. Use of imaging to assess patients with muscle disease. Curr Opin Rheumatol. 2004;16:678-683.
37. Mercuri E, Jungbluth H, Muntoni F. Muscle imaging in clinical practice: diagnostic value of muscle magnetic resonance imaging in inherited neuromuscular disorders. Curr Opin Neurol. 2005;18:526-537.
38. Lampa J, Nennesmo I, Einarsdottir H, Lundberg I. MRI guided muscle biopsy confirmed polymyositis diagnosis in a patient with interstitial lung disease. Ann Rheum Dis. 2001;60:423-426.
39. Sekul EA, Chow C, Dalakas MC. Magnetic resonance imaging of the forearm as a diagnostic aid in patients with sporadic inclusion body myositis. Neurology. 1997;48:863-866.
40. Jungbluth H, Sewry CA, Counsell S, et al. Magnetic resonance imaging of muscle in nemaline myopathy. Neuromuscul Disord. 2004;14:779-784.
41. Dwyer BA, Mayer RF, Lee SC. Progressive nemaline (rod) myopathy as a presentation of human immunodeficiency virus infection. Arch Neurol. 1992;49:440.
42. Deconinck N, Laterre EC, Van den Bergh PY. Adult-onset nemaline myopathy and monoclonal gammopathy: a case report. Acta Neurol Belg. 2000;100:34-40.
43. Bayry J, Thirion M, Misra N, et al. Mechanisms of action of intravenous immunoglobulin in autoimmune and inflammatory diseases. Neurol Sci. 2003;24(suppl 4):S217-S221.
44. Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747-755.
45. Authier FJ, Chariot P, Gherardi RK. Skeletal muscle involvement in human immunodeficiency virus (HIV)-infected patients in the era of highly active antiretroviral therapy (HAART). Muscle Nerve. 2005;32:247-260.
